On this page
-
The disease
-
Routine prevention activities
-
Surveillance objectives
-
Data management
-
Communications
-
Case defintion
-
Laboratory testing
-
Case management
-
Environmental Evaluation
-
Contact management
-
Special situations
-
References and additional sources of information
-
Appendices
-
Jurisdictional specific issues (NSW)
1. The disease
Infectious agents
The hepatitis A virus (HAV) is classified as a hepatovirus of the Picornaviridae family. It is a non‑enveloped, single-stranded, linear ribonucleic acid (RNA) virus.1 HAV displays a high degree of genetic conservation and only a single serotype of HAV exists. Overall there are six HAV genotypes consisting of three that infect humans (I-III) and three simian (monkey) strains (IV-VI).2,3 As the human HAV strains are closely related antigenically, immunity induced by a particular strain is believed to provide protection against all relevant human HAV strains.4
Reservoir
The reservoir of HAV is humans, and rarely chimpanzees and other primates.5 There is no knowledge of human susceptibility to simian HAV.6,7 HAV is resistant to low pH, heat and freezing temperatures. The virus can persist in faeces, water and soil for a prolonged period.1,5
Mode of transmission
Hepatitis A is transmitted almost entirely by the faecal-oral route. Transmission may occur through:
- ingestion of food that is not further cooked after being contaminated at any point during cultivation, harvesting, processing, distribution or preparation (usually by an infectious food handler)8
- consumption of drinking water that is not treated after contamination
- consumption of faecal material transferred from contaminated surfaces or the hands of an infectious case, and
- during sexual activity.
Children under six years of age are particularly effective transmitters of hepatitis A, as they are less likely to develop symptoms while infectious.9 Concurrence of diarrhoea allows transmission to occur more readily.
In Australia, outbreaks have previously been linked to but not limited to:
- consumption of fresh and frozen berries, frozen pomegranates, contaminated oysters, mussels and other shellfish, semi-dried tomatoes, lettuce and shallots
- settings such as childcare centres, residential facilities for the disabled, and Aboriginal and Torres Strait Islander communities (especially prior to the introduction of funded vaccination for Indigenous children living in remote areas), and
- groups such as infectious food handlers, men who have sex with men, people who use / inject illicit drugs and homeless youth.
From 2009-2014, 53% (829/1,562) of notified cases in Australia were in people who had acquired the infection during overseas travel.10
Blood-borne transmission of HAV is rare because hepatitis A viraemia is transient and does not last10 as long as faecal excretion and the concentration of virus in blood is low. Nevertheless, transmission of hepatitis A through blood and blood products has occurred, in particular among haemophiliacs receiving factor VIII concentrates.11 Blood-borne spread may be a mechanism of transmission among intravenous use of drugs among people who use / inject illicit drugs, but poor hygiene has also been implicated.12 Saliva has not been shown to be a source of infection.
Incubation period
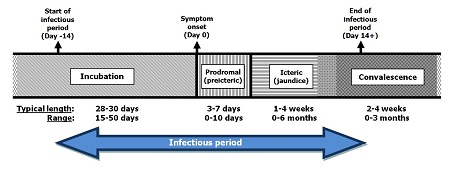
The incubation period averages 28 to 30 days, with a range of 15 to 50 days.5 See Figure 1 and Figure 2 below.
Figure 1: Stages of hepatitis A illness and length of each phase
Text alternative
Infectious period:
- Start of infectious period: 14 days prior to symptom onset (Day -14)
- Incubation period: Day -14 to symptom onset (Day 0)
- Typical length: 28 to 30 days
- Range 15 to 50 days
- Symptom onset: Day 0
- Prodromal (preicteric) phase: Day 0 until icteric phase
- Typical length: 3 to 7 days
- Range: 0 to 10 days
- Icteric phase: onset and evidence of jaundice
- Typical length: 1 to 4 weeks
- Range: 0 to 6 months
- End of infectious period: 14 days after symptom onset (Day 14+)
- Convalelescence: from end of Icteric phase
- Typical length: 2 to 4 weeks
- Range: 0 to 3 months
Figure 2: Clinical, virological and serological findings in uncomplicated acute hepatitis A13
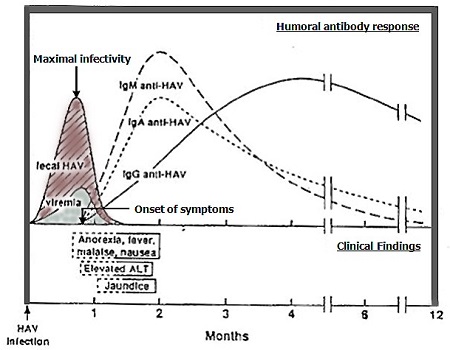
Text alternative
Humoral antibody response
- Peak titres in stool coincides with maximal infectivity, in the one to two weeks before the onset of jaundice (or peak alanine transaminase [ALT] in the absence of jaundice)
- Faecal shedding decreases significantly by 7 to 10 days after the onset of jaundice (or peak ALT)
- IgM anti-HAV begins to rise from this time tends to peak approximately 2 weeks after infection
- IgG anti-HAV rises slower, and typically peaks 4 weeks after infection
- IgM anti-HAV, IgA anti-HAV and IgG anti-HAV will decline over time
- IgG anti-HAV generally remains elavated, and a detection without elevated IgM anti-HAV may indicate previous infection
Period of acquisition
The difference between the minimum time from exposure to the virus and development of symptoms (15 days) and the maximum time from exposure to the virus and development of symptoms (50 days) is the period of acquisition (or exposure period). This is commonly used in outbreak case definitions and for separating locally acquired from overseas acquired infections.
Infectious period
Cases are considered infectious from two weeks before the onset of prodromal symptoms to either one week after the onset of jaundice (if it occurs), OR two weeks after the onset of prodromal symptoms (if jaundice does not occur). Peak titres of HAV in the stool, and therefore maximal infectivity, is highest one to two weeks before the onset of jaundice (or peak alanine transaminase [ALT] in the absence of jaundice) and faecal shedding usually has decreased significantly by 7‑10 days after the onset of jaundice (or peak ALT).5,14 See Figure 1 and Figure 2 above.
Faecal shedding of HAV may continue for several months in infants and children, in the immunosuppressed and in cases with prolonged cholestasis or relapsing illness.14,15 Faecal shedding and viraemia have been reported to persist for longer than previously supposed in immunocompetent patients (with a median period of detection after disease onset of 81 days and 42 days respectively).11 Positive faecal HAV results may not be indicative of viable virus or an infectious state especially at low counts;16 however a high level of faecal shedding (occurring for a median period of 36 days after symptom onset), can be infectious as demonstrated through the successful inoculation of virus into animals.11 For this reason, it is important to advise patients to exercise good hygiene and precautions, even after the apparent infectious period is over.
Clinical presentation and outcome
The likelihood that symptoms will follow infection increases with age. Jaundice occurs in only a small proportion of infants and young children, but appears in a majority of adults.
The usual clinical presentation of HAV infection in adults is acute onset of prodromal symptoms including fatigue, malaise, anorexia, nausea, vomiting, fever and abdominal discomfort, followed a few days later by dark-coloured urine, light-coloured stools, jaundice and pruritus. The two most common physical findings are jaundice and hepatomegaly.17 The prodromal symptoms usually diminish when jaundice appears. Jaundice typically peaks within two weeks of symptom onset and there is gradual recovery thereafter.17 Approximately 70-80% of susceptible adults and older children have symptomatic HAV infection.18 Symptomatic hepatitis occurs in around 30% of infected children under six years old, some of whom become jaundiced.18
Three atypical clinical manifestations of HAV-associated illness are recognised: prolonged cholestasis, relapsing hepatitis and extrahepatic disease.15 Prolonged cholestasis causes jaundice and pruritus for up to three months. A relapsing form of hepatitis is observed in up to 20% of patients and follows apparent full recovery. HAV infection can also rarely trigger autoimmune hepatitis and extrahepatic disease such as a fading rash, arthritis, glomerulonephritis, neurological or blood disorders.15
Persons at increased risk of severe disease
Older persons, the immunosuppressed, people with chronic liver disease, liver transplant recipients and those with chronic hepatitis B and C infection are more likely to have severe manifestations of hepatitis A illness making prevention of infection in these groups particularly important.1,4,19,20 Mortality is highly correlated with these risk factors. The case-fatality rate of hepatitis A varies from 0.1% among children <15 years of age to 2.1% among adults aged ≥40 years, usually as a result of fulminant hepatitis.1 Fulminant hepatitis is a rare consequence of HAV infection and has a high fatality rate without liver transplantation.21
Persons at increased risk of infection
The people with increased risk of infection include those who are unimmunised and:
- are household or sexual contacts of infected persons
- travel to or live in countries where hepatitis A is common (all countries of the world aside from Western Europe, Australia, New Zealand, Canada, the United States, Japan, the Republic of Korea, and Singapore)22
- are exposed to overcrowded, unsanitary areas with inadequate sewage treatment
- use illegal drugs, whether injected or not
- are men who have sex with men (MSM)
- are sex workers
- are attendees or employees of childcare facilities4
- are residents or staff of residential care facilities
- are carers of, or persons with developmental disabilities5
- have clotting factor disorders such as haemophilia (and need regular blood products)5,14
- are associated with a prison or remand centre.
Disease occurrence and public health significance
Hepatitis A was probably endemic in many parts of Australia up to the first half of the 20th century but the incidence of infection has subsequently declined as for many other developed countries due to improved water supply, hygiene and sanitation.22 A reduction in prevalence creates susceptibility to illness in older age groups which are also at risk of more severe disease.1 An Australian study which examined cross-sectional samples of stored sera taken at three time points over a 20-year period (from 1988), showed a general increase in seropositivity with increasing age.23 A seroprevalence study in Australia in 1998 estimated that 38% of the population had evidence of previous infection (varying from 70% in people aged 60-69 years to 10-20% in persons aged less than 20).24 The prevalence rates of hepatitis A have steadily declined since then. The majority of the community is now vulnerable to infection should exposure occur.
In 2017, there were 216 notifications of hepatitis A infection Australia-wide at a rate of 0.9 case per 100,000 population, with the majority of cases occurring in New South Wales, Victoria and Queensland.10 Most cases are sporadic and acquired from overseas travel, or due to small outbreaks linked to household or other close contact with infected cases. Foodborne sources may be an important cause of sporadic cases with an unidentified exposure.8,25
Australia had an outbreak of Hepatitis A emerge in several jurisdictions in 2017 with predominantly locally acquired cases in men who have sex with men (MSM). Most confirmed outbreak cases were male adults with the majority reporting male-to-male sexual contact during their incubation period. Other outbreak cases have occurred in PWID and some homeless individuals. Outbreak cases are genetically very similar to a widespread outbreak of hepatitis A in Europe, also associated with MSM. There is underreporting of hepatitis A illness to health departments due to mild or asymptomatic cases not developing characteristic symptoms, some patients not seeking care, and clinicians not notifying all cases to surveillance networks.25,26 These difficulties may be compounded in hard to reach risk groups (such as intravenous use of drugs by people who use / inject illicit drugs).26,27
Hepatitis A has been endemic in many Aboriginal and Torres Strait Islander communities in the recent past with notification rates of up to 22 cases per 100,000 population, attributed to environments with poor sanitation and inadequate safe water supply.28 A vaccination program for Aboriginal and Torres Strait Islander children was introduced in north Queensland in 1999, resulting in a 92% decrease in the number of reported cases in all children in the period 2000–2003. As a result, the national government funded a vaccination program for all Aboriginal and Torres Strait Islander children aged ≤2 years in Queensland, South Australia, Western Australia and the Northern Territory since 2005, which contributed substantially to a decline in notifications.4,28,29 In 2014, Indigenous status was known for 96% (222/231) of hepatitis A notifications, of which four 4 cases identified as Aboriginal or Torres Strait Islander.10
Outbreaks from common source infections are infrequent but have the potential to dominate regional incidence patterns and require intensive public health efforts to control.5 an outbreak due to HAV-contaminated clams in Shanghai, China, in 1988 infected >300,000 people.1 In 2009, a multistate outbreak in Australia due to imported semi-dried tomatoes led to a doubling of national hepatitis A incidence from the previous year (to 563 cases or 2.6 cases per 100,000 population).30 In 2015, outbreaks in Australia and New Zealand were associated with imported frozen berries.29 More recently in 2018, 26 people were affected by a national hepatitis A outbreak linked to imported frozen pomegranates.31 The risk of similar outbreaks in the future remains, due to the importation of foods from HAV-endemic countries.32
2. Routine prevention activities
The prevention of hepatitis A rests largely on the provision of disinfected potable water, adequate hand-washing facilities, safe sewage disposal, hygienic handling of food throughout the chain of production to consumption, and vaccination of selected cohorts.
Hepatitis A vaccine is highly effective and recommended for people at greater than background risk of getting the disease or severe illness (refer
Section 1: Persons at increased risk of disease). The expanded vaccination program since 2005 in Aboriginal and Torres Strait Islander children residing in the Northern Territory, Queensland, South Australia and Western Australia has contributed significantly to a decline in notifications.4,29
For more details on immunisation and at risk groups, refer to
The Australian Immunisation Handbook.4
3. Surveillance objectives
- To prevent further transmission of infection from cases
- To implement timely exclusions in high risk cases
- To prevent disease in exposed contacts especially those at risk of severe illness
- To rapidly identify and control common sources of infection in outbreaks
- To monitor the epidemiology of hepatitis A and inform public health policies including the development of better prevention and response strategies, and
- To report on progress towards national strategies and evaluate the effectiveness of public health programs and other interventions aimed at reducing infection.
4. Data management
Within one working day of notification, enter probable and confirmed cases of HAV infection into the jurisdictional notifiable disease database. Ensure that information on Aboriginal or Torres Strait Islander status of cases is collected and entered, and that the place of acquisition and source of infection is entered when known.
5. Communications
Both confirmed and probable cases should be notified within one working day.
Notify the Communicable Diseases Branch (CDB) about each case, including the person’s age, sex, date of onset of symptoms and/or date of diagnosis, laboratory status, suspected source of infection, risk factors for contracting the disease, severity of illness, and any links to other cases.
6. Case definition
The case definition may have been updated since the publication of this guideline. Please check the
case definitions on the Australian Department of Health’s website for the latest version.
Confirmed case
A confirmed case requires either
- laboratory definitive evidence,
or
- laboratory suggestive evidence and clinical evidence,
or
- laboratory suggestive evidence and epidemiological evidence.
Probable case
A probable case requires clinical evidence
and epidemiological evidence.
Laboratory definitive evidence
Detection of hepatitis A virus by nucleic acid testing.
Laboratory suggestive evidence
Detection of hepatitis A-specific IgM, in the absence of recent vaccination.
Clinical evidence
- Child less than five years of age,
or
- (Acute illness with discrete onset of at least two of the following signs and symptoms:
- nausea
- malaise
- abdominal discomfort
- loss of appetite
- fever,
and
- Onset of dark urine or jaundice or alanine transaminase (ALT) ten times the upper limit of normal.)
Epidemiological evidence
- Contact between two people involving a plausible mode of transmission at a time when:
- one of them is likely to be infectious (from two weeks before the onset of symptoms to a week after the onset of jaundice),
and
- the other has an illness that starts within 15 to 50 (average 28 - 30) days after this contact,
and
- At least one case in the chain of epidemiologically linked cases (which may involve many cases) is laboratory confirmed.
Case definitions can be found on the Department of Health’s website.
7. Laboratory testing
Testing guidelines
In patients not recently vaccinated for hepatitis A, diagnosis is largely established by the presence of IgM anti-HAV antibodies in combination with clinical and/or epidemiological evidence of HAV infection. IgM antibodies usually become detectable before the onset of clinical symptoms and persist for ³4 months in most persons (refer Figure 2).1,33 Probable cases with negative IgM results from early specimens should be retested in four to seven days. Routine testing for IgM antibodies in asymptomatic people is not recommended due to the high rate of false positive results in those without an appropriate clinical presentation.34
Anti-HAV IgG antibodies are markers of prior exposure to the disease or immunisation, and they persist for life after infection. Although useful for identifying persons who are currently immune to HAV infection, they are not a useful indicator of recent infection. Post-vaccination serologic testing for seroconversion does not provide meaningful results.
Although non-specific, abnormal liver function tests (LFTs) are useful in supporting a diagnosis of viral hepatitis, and are used in conjunction with jaundice to establish clinical evidence of HAV infection, and are a sensitive marker of severity.12,17,35-37 An acutely raised ALT can be a marker of infectivity as it peaks and falls in approximate alignment with HAV RNA levels.8,11
Nucleic acid testing of blood and stool, both qualitative testing and genotyping, is definitive for diagnosis14 but is only available in a few reference laboratories.33 Genotyping is essential for investigating common source outbreaks and public health surveillance.14,30 The PHU should arrange for the primary pathology laboratories to forward all positive serology specimens to the [relevant state] reference laboratory for genotyping to allow rapid epidemiological investigation.
8. Case management
Response times
Begin the follow-up investigation within one working day of notification of a probable or confirmed case.
Response procedure
Case investigation
The response to a notification will normally be carried out in collaboration with the case’s treating doctor. Regardless of who does the follow-up, PHU staff should ensure that action has been taken to:
- confirm the onset date and symptoms of the illness
- confirm results of relevant laboratory tests, or recommend the tests be done
- find out if the case, or relevant care-giver, has been told the diagnosis before the interview
- inform the treating doctor of case investigation and management of contacts
- review case and contact management, ensuring that relevant exclusions have been made
- determine the likely source of infection
- assess the number of contacts requiring prophylaxis, and
- ensure proper control measures are taken to prevent further spread.
Exposure investigation
Information regarding history of prior immunisation and of exposures during the period of acquisition (15 to 50 days before onset of symptoms) should be sought, including travel (by the case or household contacts) to countries with endemic hepatitis A. If the case has not travelled to a country with endemic hepatitis A, obtain information on the following risk factors:
- household and sexual contacts who have had an illness consistent with hepatitis
- illicit drug use whether injected or not
- anal intercourse, sex work, or male-to-male sexual contact
- restaurants or social gatherings where the case has eaten or worked
- consumption of raw or partially cooked, fresh or frozen fruit or vegetables, or ready-to-eat food, or shellfish
- all sources of drinking water and ice that were used
- recreational water exposure
- exposure to sewage, or failed sewage disposal systems
- attendance or employment in child care services, a residential or aged care facility, or a correctional facility by the case or household contacts
- hospitalisation, and/or transfusion of blood products, or
- whether the case is a health care worker (HCW) or food handler.
Recognising foodborne transmission using routine surveillance data may be difficult because cases may have difficulty recalling food histories during the two - seven weeks before illness. Cases may accrue gradually, a food item (such as in a delicatessen) may be contaminated at one end and not the other and, therefore, may not infect everyone who consumes it. Foodborne outbreaks may be widely distributed across states or even nations making it hard to pinpoint contaminated ingredients, some exposed persons may have unrecognised HAV infection, and others may have pre-existing immunity.8
Case treatment
Treatment is the responsibility of the case’s medical practitioner. There is no specific therapy for HAV infection so care is mainly supportive. This includes bed rest, antiemetics, fluids, and avoidance of hepatotoxic substances including alcohol and paracetamol if possible.17,36,38 Up to 20% of cases may require hospitalisation and cases with fulminant hepatitis may require liver transplantation.17
Education (refer Appendix 3: Hepatitis A Fact Sheet)
The case or relevant care-giver should be informed about the:
- nature of the infection
- mode of transmission
- period of infectivity
- (low) incidence of severe disease and complications
- safe hygiene practices
- exclusions and their rationale
- process of PEP for those who may become exposed, and
- hygienic practices, particularly washing hands before preparing food and drink, and after going to the toilet.
Isolation and restriction
While in the infectious period, which can be defined as:
- from two weeks before the onset of the prodrome to at least seven days after the onset of jaundice; or
- from two weeks before the onset of the prodrome to 2 weeks after the onset of symptoms if there is no jaundice; or
- for asymptomatic cases, estimated using the timing of contact with the source if known (such as contact with an index case or consumption of contaminated food) and with consideration of the laboratory test results.39 If infectious period cannot be estimated, consider convening an expert panel to decide.
Cases should:
- not donate blood
- not prepare or handle ready-to-eat food or drink for consumption by other people
- not have sex
- not provide personal care to others
- not attend childcare, preschool, primary school or work that could put others at risk
- be isolated as much as is practicable if living in a residential or aged care facility, or correctional facility, and ideally be placed in a single room with ensuite, or have a dedicated bathroom
- not share drugs or drug paraphernalia, and
- not share utensils, towels or personal items with others.
Active case finding
Where cases are identified in settings such as child care facilities, schools and residential care facilities where transmission might be expected to occur, work with health care providers and administrators to identify other possible infectious cases. Refer to
Section 10: Contact Management and
Section 11: Special Situations for more information on case finding.
9. Environmental evaluation
Viral survival characteristics
Person to person transmission or via fomites are common modes of hepatitis A transmission. HAV is resistant to degradation by environmental conditions, a property that allows its maintenance and spread within populations.40 The virus can remain infectious for at least one month at room temperature on environmental surfaces, and transfer on fomites may be important in some settings (e.g. on toys in child care centres).8,12 HAV remains on hands for ≥4 hours, and water rinsing alone reduces the amount transferred to lettuce by 10- to 100-fold.8 HAV has been found to survive in experimentally-contaminated fresh water, seawater, wastewater, soils, marine sediment, live oysters, and cream-filled cookies. It is resistant to heat (survives at 700C for 10 mins), acid treatment and freezing, but can be inactivated by formalin and chlorine.41,42 Hypochlorite-based disinfectants and cleaning products can neutralise the virus.
Water sources
Drinking water is a potential source of HAV infection if there is opportunity for faecal contamination particularly from a failed sewage disposal system.8 Water contamination is also important for shellfish which can filter several litres of sea water daily and bioaccumulate HAV to much higher concentrations than surrounding water (up to 100-fold).8,43 Outbreaks from shellfish consumption may result from inappropriate or illegal harvesting near known sources of sewage, and from discharge of sewage from fishing boats.
Where an unexpected cluster is reported, an evaluation may include review of water treatment procedures, bacteriological quality, sewage disposal systems, waterbeds where shellfish are harvested, and polymerase chain reaction (PCR) testing of shellfish samples. Specific methods to detect enteric viruses may be necessary as water and shellfish with low coliform counts (a common measure of faecal contamination) have been shown to contain viable HAV, and on occasion have caused outbreaks of hepatitis A.8 Chlorination is an effective method of controlling HAV in drinking water sources.
Food contamination
Produce might be contaminated by the hands of infected workers or children in the field, by contact with HAV-contaminated water during irrigation or rinsing after picking, or during processing.8 Common methods used for microbiological quality control of food do not reliably detect virus contamination.25 Providing sanitary facilities for field workers, using chlorinated or safe water to rinse produce or for ice for packing, and keeping children away from areas where food is harvested, may reduce the risk of contamination.8
10. Contact management
Identification of contacts
The purpose of public health intervention/ contact management is to reduce the risk of further transmission and clinical disease among those people who were contacts of the case during the case’s infectious period. In countries such as Australia with low prevalence of immunity to HAV, explosive epidemics of hepatitis A can arise from solitary sources.44 Key contacts are those people whose work or lifestyle can expose many others to the virus, and those at risk of severe disease. Key contacts therefore include:
- Food handlers and workers who handle ready-to-eat food anywhere along the cultivation to food preparation pathway
- People who share illicit drugs and drug paraphernalia
- Sex workers
- MSM, and
- Childcare workers and HCWs.
Persons aged >50 years, the immunosuppressed, and those with chronic liver disease (particularly due to hepatitis B or C) are at higher risk of severe disease.
Contact definition
The following is a general list of persons considered to be contacts if they were exposed to cases during the infectious period (refer
Section 1: Infectious period):
- Immediate family, household members and sexual partners, including people who stayed and shared the primary household facilities with the case
- Persons who consumed ready to eat food or drink prepared by the case
- If the case wears nappies, persons who provided direct care to the case
- If the case attends child care or preschool, other children and adults in the same classroom or care group, or those who share the same toilet may be considered house-hold like
- Those who shared intimate personal items or drug equipment with the case.
To determine the time period for contact identification for asymptomatic cases, refer to Section 11.
Prophylaxis
(see
Appendix 1).
In the absence of reliable self-reported or documented evidence of past infection or immunisation, PEP should be offered to contacts within two weeks of onset of symptoms in the index case to household contacts and other contacts with ongoing or intermittent household-like exposure.
Intermittent household-like contacts [scenarios]
Scenario 1
A childcare worker with confirmed HAV infection worked every day for the two week infectious period prior to the onset date of prodrome symptoms (onset date). Children in direct care of this worker were "intermittently exposed" during this period only on the days they attended the centre.
PEP should be offered to these children as soon as possible, and
- no later than 14 days after onset of prodrome symptoms in the childcare worker, if the last contact with the worker is on or around the onset of prodrome symptoms, or
- no later than 14 days after the last contact with the childcare worker if the only contact with the worker is prior to the worker’s prodrome symptom onset (refer Figure 3).
Figure 3. PEP timeline scenario 1

Text alternative
- start of infectious period: day 1
- contacts intermittently exposed (e.g. day 4, day 7, day 11) in lead up to onset of prodrome
- onset of prodrome in case on day 15; case's last day of work (owing to onset of symtoms)
- onset of jaundice: day 19
- end of infectious period: day 26
Provide PEP as soon as possible, but no later than 14 days after last contact occurred (i.e. no later than day 25, for contacts whose last exposure was on day 11; no later than day 21 for contacts whose last exposure was day 7).
Scenario 2
A sexual contact of confirmed hepatitis A case, who doesn't live in the same household as the case, had intermittent sexual contact during the case's infectious period.
PEP should be offered to the sexual contact as soon as possible, and no later than 14 days after onset of prodrome in the case (refer Figure 4).
Figure 4. PEP timeline for scenario 2

Text alternative
- start of infectious period: day 1
- contacts intermittently exposed (e.g. day 4, day 7, day 11, day 14) in lead up to onset of prodrome
- onset of prodrome in case on day 15
- further contact exposure (e.g. day 18)
- onset of jaundice: day 19
- further contact exposure (e.g. day 21)
- end of infectious period: day 26
Provide PEP as soon as possible, but no later than 14 days after onset of prodome in case (i.e. no later than day 29).
The available interventions for contacts include normal human immunoglobulin (NHIG, also called gamma globulin) and monovalent inactivated hepatitis A vaccine. Traditionally, PEP for hepatitis A contacts consisted of administration of NHIG, but in recent years, use of vaccine has gained favour.22 High quality clinically relevant evidence indicates that monovalent hepatitis A vaccine has similar efficacy to NHIG for PEP, at least for people aged between 1 and 40 years.45,46 The vaccine also has the advantage of conferring long-term immunity, being readily available, and being more effective at interrupting longer lasting outbreaks with possible multiple exposures.9,19
For asymptomatic cases, refer Section 11.
Passive immunisation
NHIG is a preparation of pooled antibodies and its use worldwide is declining due to insufficient concentrations of anti-HAV IgG, high cost, and limited duration of protection.1 However, in Australia adequate concentration of anti-HAV IgG is ensured. Supply of NHIG is by local arrangement within each jurisdiction. NHIG should be given in a single intramuscular injection at the following dosage:
| <25 kg | 0.5 ml |
|---|
| 25-50 kg | 1.0 ml |
|---|
| >50 kg | 2.0 ml |
|---|
The dose is effective for one to two months but can be tripled to 0.06 ml/kg if required, to offer longer term protection for up to 5 months.47 NHIG may not prevent excretion of HAV, so those given NHIG may still transmit the virus, even if they do not develop clinical illness. The administration of NHIG can interfere with the immune response from live virus vaccines such as measles, mumps and rubella (MMR), and varicella, and the childhood immunisation schedule in particular may require adjustment. For detailed advice, consult latest edition of
The Australian Immunisation Handbook.
Active immunisation
A single dose of monovalent hepatitis A vaccine gives protection to most recipients within two weeks. A two-dose course six months apart is recommended for long-lasting protection. Persons given a single dose for PEP should be advised to complete the course through their general practitioner (GP) as the vaccine is not subsidised. Combination hepatitis A/B and hepatitis A/typhoid vaccines are useful for pre-exposure prophylaxis in selected individuals but are not recommended for PEP due to reduced vaccine HAV antigen levels. Not all vaccines are licensed for children aged down to one year of age making it important to check details in
The Australian Immunisation Handbook. Note also that recent immunisation may confuse the interpretation of follow-up serology.
Antibiotic prophylaxis
None.
PEP recommendations
PHU staff should ensure that the facility's infection control procedures are reviewed to determine the likelihood of disease
| Healthy contacts aged 1-40 years for whom vaccine is not contraindicated | Monovalent hepatitis A vaccine onlyb |
|---|
| Healthy contacts aged ≥ 12 months for whom vaccine is contraindicated | NHIG only |
|---|
| Contacts aged <12 months | NHIG only |
|---|
| Contacts aged ≥ 12 months with chronic liver disease or immunosuppression | NHIG and monovalent hepatitis A vaccine vaccinec |
|---|
| Healthy contacts aged >40 years | NHIG and/or vaccine (see discussion below and conduct risk assessment in conjunction with contact’s GP) |
|---|
There is variation in recommendations from public health authorities in comparable countries regarding the effective upper age limit for hepatitis A vaccine for post-exposure prophylaxis, and also regarding recommendations for those with chronic liver disease or immunosuppression. The United States of America recommend an upper age of 40 years for hepatitis A vaccine, and use of NHIG in contacts older than 40 years, based on randomised controlled trial evidence.37,48 Public Health England recommends preferential use of vaccine over NHIG up to age 60 years.39 The Public Health Agency of Canada prefers vaccine over NHIG for any age over six months.
The evidence base for these varying recommendations is the same. There is evidence of increased risk of severe disease with increasing age.49 There is also evidence that the rapidity of achieving seroprotective levels after one dose of hepatitis A vaccine decreases with age, with seroprotective levels reached after 15 days in 74%, 54%, and 30% of individuals aged 40-49, 50-59 and 60-69 years respectively.48 This compares to 92% seroconversion after 15 days in individuals younger than 40 years.50 It appears that some of the move to preferring vaccine over NHIG is concern about decreasing potency of NHIG products. This is not a consideration in Australia, as the Australian Red Cross Blood Service (ARCBS) does not release NHIG unless the antibody levels in the final product are ≥100 IU/mL [V. Hoad personal communication September 2017].
Nevertheless, many adult contacts >40 years in Australia would receive an additional benefit of longer-term protection from vaccination compared to NHIG. It is also likely that most adults 40 to 60 years who are otherwise well and are not at high risk of severe disease will mount a sufficiently rapid immune response to the vaccine to prevent disease. In such cases, PHUs may wish to undertake a risk assessment with the contact and their GP, and consider using vaccine in otherwise well adults 40 – 60 years of age in preference to NHIG. Alternately, vaccine and/ or NHIG can be administered simultaneously at different sites on the body without significant interference in the long-term level of seroprotection.36
Provision of NHIG is recommended for the immunosuppressed and people with chronic liver disease, due to a delayed or weakened immune response from vaccine alone and the risk of serious disease.9,14,51
There is international consensus that in pregnancy the theoretical risk of inactivated vaccine to the fetus is low.1,14 Live attenuated hepatitis A vaccines (currently available in China) should not be given to pregnant women.1 The risk of vaccination in pregnancy should be weighed against the risk associated with HAV infection. An immunity check on pregnant women should be performed prior to immunoglobulin administration.
There is conflicting evidence whether NHIG is effective >14 days post exposure and whether it attenuates the severity of hepatitis A infection.9,45 PEP is not indicated for contacts of sporadic cases in the school or work settings.5
Immunity screening
Immunity screening (using IgG levels) to avoid the needless administration of PEP is practiced in various Australian jurisdictions for groups with a high likelihood of previous infection (those who have spent many years in an HAV endemic country or are aged ≥ 40 years). Testing is undertaken early in the 14 days window of opportunity in exposed individuals to allow for later administration of prophylaxis. The decision for testing should be based chiefly on the expected prevalence of immunity, the cost-benefit, and the degree of risk in interfering with provision of subsequent PEP.14 There is no reported harm specific to administering vaccine or NHIG to immune persons.
Education
Provide contacts (or parents/ guardians) with a Hepatitis A Fact Sheet (refer
Appendix 4), including advice on the risk of infection and the need for PEP particularly in at-risk groups. Counsel them to watch for signs or symptoms of hepatitis occurring within 50 days of exposure and seek medical attention early if symptoms develop. Parents of infants or young children should be reminded that jaundice may not occur in the young. Advice about careful hygiene should be given, particularly about hand washing after going to the toilet and changing nappies. It is especially important that food handlers monitor their symptoms after contact with the case and seek medical attention promptly if symptoms are detected. Contacts such as food handlers and other high-risk workers who are likely exposed to hepatitis A (e.g. ate a salad prepared by the case), should voluntarily exclude themselves from settings that put others in potential exposure to HAV infection.
Isolation and restriction
According to current legislation, contacts are not subject to enforced exclusions and cannot be treated as cases. At best, contacts can be advised to voluntarily exclude themselves and to curb high-risk behaviours or work practices until the incubation period ends or, if they become cases, until their infectious period ends. Individual risk assessment should be utilised to inform contacts of the likelihood of infection and the threat they pose to others. Timely PEP will minimise the probability of contacts becoming cases.
11. Special situations
Asymptomatic cases
Suspected cases believed to be infectious but asymptomatic, such as children <5 years old should be managed with observation, PEP of contacts if indicated (e.g. as for contact management in child care centres), and with good hygiene practices. Standard precautions should be used by direct contacts for the suspected period of infectivity. Deranged LFTs can be a marker of infectiousness but they are not always altered in mild disease. It is important to seek expert advice on which laboratory tests (serum IgM, serum PCR or PCR testing of stools16) can be used to confirm asymptomatic infection in an outbreak setting. The general aim is to manage contacts based on an individual risk assessment.
A case in a child care setting
Because most HAV infections in young children are asymptomatic, illness among staff members or household contacts is often the first (and only) indication of child care centre outbreaks.12 The exclusion period for a diagnosed case should be considered. Asymptomatic cases with HAV undetectable by PCR on stool can safely return to child care. Others that may remain PCR positive in stool should be assessed on a case by case basis.
For a single case, hepatitis A vaccine or NHIG needs be administered only to classroom contacts of the index case while they were infectious. Written advice should be provided to parents and staff caring for children in other groups.
For an outbreak, hepatitis A vaccine or NHIG should be administered to all previously unvaccinated staff members and attendees of the childcare facility
and members of households who have children (centre attendees) in nappies.
An outbreak occurs when:
- HAV cases occur in two or more children or employees (without another plausible exposure source); or
- HAV cases are recognised in two or more households epidemiologically linked to the centre (without another plausible exposure source).
To quickly identify new infections, the PHU should institute surveillance for hepatitis-like illness among households connected to the centre for 50 days after onset of the last case; usually done by letter. All such households should be provided with basic information about HAV, and be instructed to contact the PHU immediately should compatible symptoms develop.
The critical role of good personal hygiene (especially hand washing) should be reviewed with childcare centre staff. Staff involved in food handling, should not be involved in changing nappies during the same shift or day. Affected centres should be discouraged from accepting new children for 50 days after onset of the last case, unless hepatitis A vaccine or NHIG is given before admission. Transferring children to other centres should be discouraged during this period. All surfaces and toys in affected classrooms should be cleaned and sanitised daily. Toys that can’t be washed should be temporarily removed.
A case in a school, hospital, or work setting
Hepatitis A PEP is not routinely indicated when a single case occurs in a school or work setting and the source of infection is outside the school or work setting. Similarly, when a person who has hepatitis A is admitted to a hospital, staff members should not routinely be administered HAV PEP. Careful hygienic practices and standard precautions should be emphasised. Appropriate PEP should be administered to persons who have close contact with index patients if an epidemiologic investigation indicates HAV transmission has occurred among students in a school, among patients or between patients and staff members in a hospital, and circumstances suggest that further protection over and above improvements in hygienic practices is warranted.
Where the index case is a HCW (infected via another source) a careful risk assessment is required to assess the risk to other HCWs and patients, including: an audit of infection control practice, investigation of the type of care provided (feeding, toileting, etc.), food/bathroom facilities shared with other HCWs, and the potential infectiousness of the case (diarrhoea at work, stage of infection). PEP may be required for non-immune staff and patients.
A case in a food handler (see
Appendix 2)
Food handlers are not at increased risk for HAV infection because of their occupation, but are noteworthy because of their critical role in common-source foodborne HAV transmission.14 Roughly 8% of the working population are food handlers and around the same proportion (8%) of all hepatitis A cases occur in food handlers.8,12 Most food handlers with hepatitis A do not transmit the virus to others. The public health response is based on a careful case-by-case risk assessment, which may require on-site visits (by environmental health officers and [the NSW Food Authority]), independent verification of hygiene practices, and detailed process and document review. The evaluation should estimate the number of potentially exposed individuals and their risk of severe or asymptomatic disease, as well as cover media and political considerations.
Risk assessment in food handlers should incorporate the following principles:
- make every possible effort to obtain accurate information
- exercise considerable judgment about the accuracy of information received, especially the consistency of hygiene information received from different sources
- consider the facility’s food inspections records while under its current management
- determine whether the manager has had food safety training and applies it through employee training, supervision and hazard control systems at the facility. Good practices include:
- management supervising and inspecting food handling practices of all shifts on a routine basis
- training that addresses personal hygiene and supervision of hand washing practices
- management has a routine practice of evaluating employee hygiene practices, such as washing hands upon entering a food preparation area and on leaving a restroom
- hand washing facilities are checked frequently each day for adequate supplies, and operational records are kept
- high risk food handling tasks of ready-to-eat food are designed so that direct handling of food and cross-contamination are minimised
- an effective management policy is in place to encourage employees to report illnesses and not to work with symptoms that could indicate a communicable disease (e.g. diarrhoea or vomiting), and that sick leave and exclusion records are well maintained.
Consider follow up of patrons if the food handler, while infectious, directly handled food that was not subsequently cooked prior to serving
and had diarrhoea or poor hygienic practices
and patrons can be identified and provided with PEP within two weeks of exposure. Use of PEP should be considered in institutional settings where multiple exposures among patrons may have occurred. PHU staff must work in close collaboration with the [NSW Food Authority] in managing the risk to other staff and patrons.
Risk to other food handlers and patrons from a food handler diagnosed with HAV
Food handlers can transmit HAV to patrons and co-workers through contaminated food, and possibly utensils or surfaces. If the investigation shows that other food handlers at the facility are at risk because they either ate food prepared by the case, or because they shared toilets or washing facilities with the case, then PEP should be provided to those who are unimmunised. Where other cases are suspected among food handlers, blood should be collected (with consent) for testing.
Risk communication – patrons
It can be difficult to identify all people who may have been exposed to food prepared by a case. It is therefore important to evaluate the degree of risk to patrons by assessing the risk behaviours of the case. Where a risk is identified, there are two primary reasons to alert patrons:
- to provide PEP to potentially exposed individuals and prevent illness in them
- to warn and educate persons who may be already incubating the infections (and their doctors) about their exposure, in order to facilitate rapid diagnosis and to prevent a subsequent generation of cases. (Public announcements can be worthwhile even if it is too late to offer PEP to exposed individuals).
These measures can be readily applied in a setting where at-risk groups are easily located, such as a school, childcare centre or private home. For restaurants and sandwich shops it sometimes becomes necessary to notify those at risk through the news media or other forms of public announcements. The food service facility operators should be counselled about their responsibility to protect the public’s health and the need to cooperate in public alerts.
Risk communication – general public
After a careful risk assessment, the PHU should alert the general public about a food outlet or infectious food handler (through the news media) when there is evidence of infection in other food handlers or patrons at the site,
and there are significant concerns about food safety breaches
and if patrons cannot be easily contacted within two weeks of exposure. Suspected common-source outbreaks unrelated to food preparation should be clearly communicated. The PHU should consult with the communicable disease branch (CDB) staff, [the NSW Food Authority] and media units before going public. The CDB may convene an expert panel to advise in difficult cases.
General principles for decision-making
(Refer
Appendix 2).
The pivotal decision points for contact management of a HAV-infected food handler relate to:
- whether the food handler was working during the infectious period and had contact with high risk ready-to-eat food
- if patrons or staff had repeated exposures
- if the food handler had good hygiene practices and was free of diarrhoea during the infectious period
- if the current management has good food service training and there is an effective hazard control system in the facility
- if patrons/ contacts can be identified and contacted within two weeks
- if there is evidence of infection in other food handlers or patrons.
Good hygiene standards include: the case always uses gloves or utensils appropriately; changes gloves when food preparation is interrupted; meticulously washes hands after toileting and before food preparation; has access to adequate and clean toilet facility; management has undertaken approved training and implements an approved hazard control system; hand washing facilities are clean, well-stocked (with soap and disposable towels), accessible and well-utilised; food is adequately heated or refrigerated and not left out for extensive periods; and surfaces are regularly cleaned and sanitised.
High risk situations arise when there is a history of multiple exposures by staff or patrons and where a facility’s or a food handler’s hygiene standards are questionable. In these circumstances, consider communicating the incident through the media, offering of PEP to contacts, and implementing exclusions.
If there is evidence of infection in other food handlers or patrons, irrespective of hygiene standards in the facility or staff, re-evaluate the risk to the public and undertake a thorough investigation of the premises. This would include corroborating evidence with a range of stakeholders, review of illness registers and past inspection/audit records, search for council orders or fines and complaints to council, and the sending away of multiple specimens for testing.
Low risk scenarios for ongoing transmission from an infected food handler are ones where there is no evidence of hygiene breaches (cross-contamination issues etc.) and no evidence of transmission of HAV infection to others. PEP should be offered to other food handlers even in this situation to prevent secondary cases in critical staff and because food handling staff may have consumed contaminated food.8
In all instances, other food handlers at the establishment in question should be evaluated to determine whether any have, or recently have had, HAV, and if it was locally acquired. Past inspection records should be reviewed and interview statements should be corroborated. The potential for breaks in proper practices should be carefully evaluated. The PHU and food service managers should monitor food handlers who are at risk of HAV for one incubation period (50 days) after their last exposure to the index case.
Opportunities for PEP are often missed either because the infected food handler did not receive a diagnosis of HAV infection until after transmission to patrons had occurred, the case was not reported to the local public health authorities, or reported food handling practices incorrectly indicated a low risk of transmission.8 PEP should not be offered to exposed persons after secondary cases have begun to occur because the two week window of opportunity for effective PEP will have elapsed, unless other infected food handlers with later symptom onsets have been identified, or prevention of tertiary cases is being considered.
Outbreak investigation
There is a different approach to investigating outbreaks of HAV illness from:
- a localised point source and
- a widely distributed food.
In the latter case, it may be necessary to undertake hypothesis generation of transmission pathways through trawling questionnaires and other tools; to communicate with neighbouring public health units, jurisdictions and national bodies; to test food and water supplies; and to test epidemiological hypotheses through analytic studies (case control and cohort). A detailed discussion of outbreak investigation is beyond the scope of this guideline. In both scenarios prompt action must be taken to determine the:
- likely source of transmission (e.g. water, distributed frozen food, shellfish etc.)
- size of the outbreak
- likely number of affected people and their risk of transmission to others
- type of facilities affected (e.g. residential and aged care facilities, supermarkets, restaurants)
- the best means to curb further spread.
Depending on the affected population and setting, control strategies may also include:
- active case finding
- genotyping and sequencing of HAV detected in clinical or food samples
- exclusion of cases and suspected cases from at-risk work
- closure of affected facilities or food outlets
- trace-back of suspected food sources
- sending alerts to doctors in the community
- conducting media alerts to the wider community
- taking other measures such as the community-wide promotion of vaccination.
Locally-acquired cases in men who have sex with men
Cases of HAV reported in MSM with no history of overseas travel during the incubation period should prompt careful questioning to identify sexual contacts who may have been the source of infection, or who may have been exposed to the case while he was infectious. Specific inquiry should be made about visits to public sex venues, use of mobile phone apps to find sexual partners, and all other regular and casual sex partners during the periods of interest.
Partners who can be identified within two weeks of contact with the case while infectious should be assessed for symptoms of HAV infection and offered PEP if not already immune. If high risk settings, or persons at higher risk from HAV, are identified, consideration should be given to:
- public alerts (targeting MSM community media), emphasising vaccination and risk reduction practices, hand washing after toileting, before eating, before preparing food or drink, after handling condoms and sex toys/equipment, and after sex
- signage or campaigns in public sex venues
- clinician alerts, emphasising early detection of cases and routine vaccination of MSM
12. References and additional sources of information
-
World Health Organization. WHO position paper on hepatitis A vaccines.
Wkly Epidemiol Rec. 2012;87:261-76.
-
Costa-Mattioli M, Ferre V, Monpoeho S, Garcia L, Colina R, Billaudel S, et al. Genetic variability of hepatitis A virus in South America reveals heterogeneity and co-circulation during epidemic outbreaks.
The Journal of general virology. 2001;82(Pt 11):2647-52. Epub 2001/10/17.
-
Robertson BH, Jansen RW, Khanna B, Totsuka A, Nainan OV, Siegl G, et al. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions.
The Journal of general virology. 1992;73 ( Pt 6):1365-77. Epub 1992/06/01.
-
Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook, Australian Government Department of Health, Canberra, 2018, immunisationhandbook.health.gov.au.
-
Spradling PR. Hepatitis, Viral. In: Heymann DL, editor. Control of communicable diseases manual. 20th ed. Washington: American Public Health Association; 2015. p. 252-74.
-
Beard MRL, S. M.;. Hepatitis A Virus (Picornaviridae). In: G.; GAWR, editor. Encyclopedia of Virology. Second ed: Elsevier; 1999.
-
Mushawar I. Viral Heapatitis: Molecular Biology, Diagnosis, Epidemiology and Control. Zuckerman AM, IK;, editor: Elsevier; 2004.
-
Fiore A. Hepatitis A transmitted by food. Clin Infect Dis. 2004;38(5):705-15.
-
Thomas L and The Hepatitis A Guidelines Group. Guidance for the prevention and control of hepatitis A infection (v1.1). Health Protection Agency (Public Health England
-
NNDSS Annual Report Working Group. Australia's notifiable disease status, 2014: Annual report of the National Notifiable Diseases Surveillance System.
Commun Dis Intell Q Rep. 2016;40(1):E48-145. Epub 2016/04/16.
-
Tjon G, Coutinho R, van den Hoek A, Esman S, Wijkmans C, Hoebe C, et al. High and persistent excretion of hepatitis A virus in immunocompetent patients.
J Med Virol. 2006;78:1398-405.
-
Oregon Health Authority. Hepatitis A Investigative Guidelines. Public Health Division 2016 [updated Jul 2016; cited 2017 13 Apr].
-
Stapleton J, Lemon S. Hepatitis A and hepatitis E. In: Hoeprich P, Jordan M, Ronald A, editors. Infectious diseases: A treatise of infectious processes. Philadelphia: Lippincott; 1994. p. 790-7.
-
Centers for Disease Control and Prevention. Chapter 9, Hepatitis A. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13 ed. Washington DC: Public Health Foundation; 2015. p. 135-47.
-
Cheney C. Atypical manifestations of Hepatitis A virus infection. UpToDate. 2014.
-
Rump B, Visser O, Te Wierik M, Vennema H. Use of PCR for detection of faecal HAV as a screening tool in an outbreak of hepatitis A in daycare centres.
Epidemiol Infect. 2013;141:549-55.
-
Lai M, Chopra S. Overview of hepatitis A virus infection in adults. 2016 [updated 17 Oct 2016; cited 2017 14 Apr
-
Quiros-Tejeira R. Overview of hepatitis A virus infection in children. 2016.
-
Advisory Committee on Immunization Practices (ACIP), Centres for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(41):1080-4.
-
Cooksley G. The importance and benefits of hepatitis A prevention in chronic liver disease patients.
J Gastroenterol Hepatol. 2004;19:S17-S20.
-
Taylor RM, Davern T, Munoz S, Han SH, McGuire B, Larson AM, et al. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes.
Hepatology. 2006;44(6):1589-97. Epub 2006/11/30.
-
World Health Organization. Evidence based recommendation for use of hepatitis A vaccines in immunization services: Background paper for SAGE discussions. Geneva: WHO, 2011.
-
Heywood AE, Newall AT, Gao Z, Wood JG, Breschkin A, Nicholson S, et al. Changes in seroprevalence to hepatitis A in Victoria, Australia: a comparison of three time points.
Vaccine. 2012;30(42):6020-6. Epub 2012/08/08.
-
Amin J, Gilbert G, Escott R, Heath T, Burgess M. Hepatitis A epidemiology in Australia: national seroprevalence and notifications.
Med J Aust. 2001;174(7):338-41.
-
Petrignani M, Verhoef L, Vennema H, van Hunen R, Baas D, van Steenbergen J, et al. Underdiagnosis of foodborne hepatitis A, the Netherlands, 2008-2010.
Emerg Infect Dis. 2014;20(4):596-602.
-
Klevens R, Liu S, Roberts H, Jiles R, SD H. Estimating acute viral hepatitis infections from nationally reported cases.
Am J Public Health. 2014;104(3):482-7.
-
Matin N, Grant A, Granerod J, Crowcroft N. Hepatitis A surveillance in England - how many cases are not reported and does it really matter?
Epidemiol Infect. 2006;134:1299-302.
-
Australian Indigenous HealthInfoNet. Summary of hepatitis A/B among Indigenous Australians. Australian Indigenous HealthInfoNet, 2008.
-
Thompson C, Dey A, Fearnley E, Polkinghorne B, Beard F. Impact of the national targeted Hepatitis A immunisation program in Australia: 2000–2014.
Vaccine. 2017;35(1):170-6.
-
Donnan E, Fielding J, Gregory J, Lalor K, Rowe S, Goldsmith S, et al. A Multistate outbreak of hepatitis A associated with semidried tomatoes in Australia, 2009.
Clin Infect Dis. 2012;54(6): 775-81.
-
NSW Health. Hepatitis A linked to pomegranate: 2018 Infectious Disease Alert
-
Holmberg S. Hepatitis A epidemiology goes global (editorial commentary).
Clin Infect Dis. 2012;54(6):782-3.
-
Public Health Laboratory Network (PHLN). Hepatitis A laboratory case definition (LCD). Canberra: Australian Government Department of Health; 2011 [updated 1 Jun; cited 2017 18 Apr]
-
Alatoom A, Ansari M, Cuthbert J. Multiple factors contribute to positive results for hepatitis A virus Immunoglobulin M antibody.
Arch Path Lab Med. 2013;137:90-5.
-
Thapa B, Walia A. Liver function tests and their interpretation.
Indian J Paediatr. 2007;74:663-71.
-
Matheny S, Kingery J. Hepatitis A.
Am Fam Physician. 2012;86(11):1027-34.
-
Hussain Z, Husain S, Almajdhi N, Kar P. Immunological and molecular epidemiology characteristics of acute and fulminant viral hepatitis A.
Virology J. 2011;8:254.
-
Jeong S-H, Lee H-S. Hepatitis A: clinical manifestations and management.
Intervirology. 2010;53:15-9.
-
England PH. Public health control and management of hepatitis A: 2017 Guidelines. 2017.
-
Stapleton JT. Host immune response to hepatitis A virus.
The Journal of infectious diseases. 1995;171 Suppl 1:S9-14. Epub 1995/03/01.
-
Zhu L, Zhang X. Hepatitis A virus exhibits a structure unique among picornaviruses.
Protein & cell. 2015;6(2):79-80. Epub 2014/11/02.
-
Leach CT. Hepatitis A in the United States.
The Pediatric infectious disease journal. 2004;23(6):551-2. Epub 2004/06/15.
-
Namsai A, Louisirirotchanakul S, Wongchinda N, Siripanyaphinyo U, Virulhakul P, Puthavathana P, et al. Surveillance of hepatitis A and E viruses contamination in shellfish in Thailand.
Lett in Apl Microbiol. 2011;53:608-13.
-
FitzSimons D, Hendrickx G, Vorsters A, Van Damme P. Hepatitis A and E: update on prevention and epidemiology. Vaccine. 2010;28(3):583-8. Epub 2009/11/21.
-
Victor J, Monto A, Surdina T, Suleimenova S, Vaughan G, Nainan O, et al. Hepatitis A vaccine versus immune globulin for post exposure prophylaxis.
N Engl J Med. 2007;357(17):1685-94.
-
Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, et al. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children.
N Engl J Med. 1992;327(7):453-7.
-
Advisory Committee on Immunization P, Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports. 2006;55(RR-7):1-23. Epub 2006/05/19.
-
Nelson NP, Murphy TV, McMahon BJ. Hepatitis A vaccination for post-exposure prophylaxis in persons aged 40 years and older.
Vaccine. 2014;32(25):2939. Epub 2014/02/18.
-
Lednar WM, Lemon SM, Kirkpatrick JW, Redfield RR, Fields ML, Kelley PW. Frequency of illness associated with epidemic hepatitis A virus infections in adults.
American journal of epidemiology. 1985;122(2):226-33. Epub 1985/08/01.
-
Van Der Meeren O, Crasta P, de Ridder M. A retrospective pooled analysis assessing the effect of age on the immunogenicity of Havrix™ in healthy adults.
Human vaccines & immunotherapeutics. 2015;11(7):1729-34.
-
Public Health Agency of Canada. Canadian immunization guide: Part 4 - Active vaccines: Hepatitis A vaccine. Canadian Government; 2016 [updated Sep 2016; cited 2017 18 Apr]
13. Appendices
-
Appendix 1: Algorithm for management of household contacts of hepatitis A
-
Appendix 2: Algorithm for case and contact management of food handlers with hepatitis A
-
Appendix 3: Hepatitis A factsheet
-
Appendix 4: Hepatitis A Public Health Unit checklist
-
Appendix 5: Questionnaire for investigation of hepatitis A cases
14. Jurisdiction specific issues
Links to State and Territory Public Health Legislation, the Biosecurity Act 2015 and the National Health Security Act 2007.
NSW Legislation
NSW Public Health Act 2010.
Hepatitis A is to be notified by:
- medical practitioners and hospital CEOs
- laboratories.
Access to normal human immunoglobulin (NHIg) under the national blood arrangements
- NHIg may be supplied under the national blood arrangements for public health disease control activities, to treat susceptible contacts of an indicated infectious disease (hepatitis A, measles, poliomyelitis or rubella), where directed by the public health units in each state and territory.
- To order NHIg for public health disease post-exposure prophylaxis, follow the instructions at the
National Blood Authority website, including completing and lodging a Normal human immunoglobulin (NHIg) order form.
- In addition, NSW Health has authorised the pre-positioning of NHIg in certain rural areas (including John Hunter, Bathurst, Orange, Dubbo, Port Macquarie, Coffs Harbour, Lismore, Tamworth, Armidale, Wagga Wagga, Goulburn, Bega, Griffith, and Broken Hill) to facilitate urgent access. The ARCBS is still charged with authorising access to that stock – this is because it may be more efficient to deliver short-expiry stock from elsewhere (generally the stock pre-positioned will be long-expiry stock).