-
Summary
-
The disease
-
Routine prevention activities
-
Human disease surveillance objectives
-
Data management
-
Communications
-
Case definition
-
Laboratory testing
-
Case management
-
Environmental risk management
-
Contact management
-
Special situations
-
References
-
Further information and resources
-
Appendices
-
Jurisdiction specific issues
1. Summary
Public health priority: High
Urgent for cases known to have recently travelled in the ZIKV-receptive area of Australia either during exposure or viraemic periods. Routine for other cases.
Case management
No specific treatment is available for ZIKV infection. Most symptomatic cases are self-limiting, with oral fluids and analgesia given acutely. Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided until dengue can be ruled out to reduce the risk of haemorrhage.
ZIKV cases should be advised not to travel to the ZIKV-receptive area of Australia and to avoid being bitten by mosquitoes capable of transmitting ZIKV for at least the first week after onset of symptoms or laboratory confirmation of infection. Pregnant women with ZIKV infection should be referred for specialist obstetric assessment.
Male cases should avoid unprotected sex during the infectious period and for at least six months after their return home, or for six months after the date that ZIKV infection was diagnosed – whichever is longer. Female cases should avoid unprotected sex, during the infectious period and for at least eight weeks after their return home, or for eight weeks after the date that ZIKV infection was diagnosed – whichever is longer.
Male cases should not donate sperm for at least six months from the time of their last exposure to or time of diagnosis, of the virus.
All cases cannot donate blood for a minimum of four months after recovery from all symptoms.
Contact management
Identify and manage pregnant women who were co-travellers or are sexual partners of confirmed or probable ZIKV cases. Infants born to mothers infected with ZIKV during pregnancy should be tested and referred for specialist paediatric assessment.
Additional contact management is required in the ZIKV-receptive area of Australia. All men and women should follow the recommendations for prevention of sexual transmission that are relevant to their circumstances (refer to Appendix 1).
ZIKV-receptive area of Australia
For the purposes of this document, the receptive area for ZIKV is considered to be the same as the dengue-receptive area (Figure 1), as defined in the Queensland Dengue Management Plan 2015-20201; mainly north Queensland.
This includes residential parts* (urban or regional) of a defined geographical region in Australia where:
-
Aedes aegypti or
Aedes albopictus mosquitoes are considered to be present
-
and local dengue transmission has occurred in the past 20 years,
or local public health and entomology authorities consider there to be a risk of sustained transmission.
* Note:
- Only urban or residential environments within the shaded areas are the potential receptive area.
- Data on vector distribution are patchy and subject to change.
Figure 1. ZIKV-receptive area of Australia
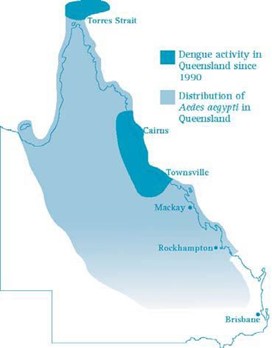
Source:
Queensland Dengue Management Plan 2015-20201.
To date there has been no known local transmission of ZIKV in the receptive area of Australia.
2. The disease
Infectious agents
Zika virus (ZIKV) is a flavivirus, closely related to dengue virus.
It was first isolated in 1947 from a rhesus monkey in the Zika forest, Uganda, then in mosquitoes (Aedes africanus) in the same forest in 19482, and in a human from Nigeria in 1952.3 There are two distinct ZIKV lineages: the African lineage and the Asian lineage, the latter of which has emerged recently in the Pacific and the Americas.4
The first outbreak of ZIKV infection identified outside of Africa and Asia, occurred on Yap Island, Federated States of Micronesia in 2007. The outbreak was estimated to have affected over 900 people and the predominant mosquito vector was
Aedes hensilli.5
In 2013, an outbreak of ZIKV infection occurred in French Polynesia, affecting more than 28,000 people.6, 7 Whilst most of those affected experienced mild symptoms, severe neurological complications were reported in a few patients.8
In 2015, ZIKV emerged in South America with widespread outbreaks reported initially in Brazil and Columbia
9, 10, with subsequent spread to many countries in South and Central America and the Caribbean.
Reservoir
Humans are the predominant host during outbreaks. Non-human primates such as monkeys are thought to maintain the virus in limited forest settings in Asia and Africa.11,12
Mode of transmission
ZIKV is transmitted to humans primarily though the bite of infective Aedes mosquitoes. It is assumed that
Ae. aegypti is the principal vector for ZIKV transmission.
Virus has been detected from mosquitoes trapped in the wild and vector competence has been shown for
Ae. aegypti11,13 and
Ae. albopictus14 ,15 Some other Aedes mosquito species are considered potential vectors (notably
Ae. africanus16,
Ae. hensilli5, 17, 18 and
Ae. polynesiensis19 ) in particular geographic locations.
Vertical transmission
Maternal-fetal transmission of ZIKV has been documented with a number of reports demonstrating ZIKV in amniotic fluid, as well as the blood and tissue of fetuses or infants born to women with ZIKV infections.
20-27
Perinatal transmission could occur by trans-placental transmission or during delivery.
Breastfeeding
To date, there are no reports of infants being infected with ZIKV through breastfeeding; however, ZIKV RNA has been detected in breast milk.28
The World Health Organization recommends breastfeeding continues, with benefits for the infant and mother outweighing any potential risk of ZIKV transmission through breast milk.29
Sexual transmission
ZIKV can be transmitted during sex by a person infected with ZIKV to their partner (female or male). Multiple instances of probable or confirmed sexual transmission have now been reported. To date almost all reports of sexual transmission have involved a symptomatic man
30-35, however, transmission from asymptomatic males to female sexual partners has also been reported,36, 37 as has female-to-male and male-to-male sexual transmission.34, 38 From these cases, it is known that the sexual transmission can occur before, during or after symptoms.
The longest reported period between symptom onset and sexual transmission is 32-41 days (based on an incubation period of 3-12 days).39 Zika virus RNA has also been found in the semen of five men more than 90 days after onset, and in one case up to 188 days after onset of infection.40-43 Viral RNA has been detected in the genital tract of women on day 11 in one, and another up to day 13 in another, and was cleared by day 17 in both women.44, 45 It is not known if ZIKV can be transmitted from other body fluids.
For recommendations to reduce the risk of sexual transmission see Sexual transmission advice under Routine prevention activities (below).
Transfusion-derived transmission
During the outbreak in French Polynesia, 3% of blood donors were found to be positive for ZIKV by PCR while asymptomatic.46 There is also evidence of ZIKV transmission from blood transfusions in Brazil.47, 48
For further information see the section on Blood donation.
Incubation period
The incubation period in a susceptible human (i.e. the intrinsic incubation period) has been reported to be between 3 and 12 days, following the bite of an infective vector.49
The extrinsic incubation period (length of time for a mosquito to become infective after ingesting ZIKV) is estimated to be 10 days. An infective vector is thought to be capable of transmitting ZIKV until it dies.
Infectious period
A person with ZIKV infection is thought to be able to transmit the virus to vector mosquitoes during the first week of their infection.
In symptomatic cases, the infectious period is likely to start 2-3 days prior to the onset of symptoms and continues up to 10 days after symptoms end.
Clinical presentation and outcome
Most (between 60 to 80%) people with ZIKV infection are thought to remain asymptomatic.
If symptomatic, infection is usually mild, and characterised by a short-lasting, self-limiting rash illness of 4–7 days duration, without complications. Exceptions are the risk of effects to the fetus in pregnant women, and the development of neurological complications (such as Guillain-Barré Syndrome (GBS)), which are discussed below.
The main symptoms of ZIKV infection are maculopapular rash, fever, arthralgia, myalgia, headache and non-purulent conjunctivitis or conjunctival injection. The rash is often itchy and centrifugal (starts on the face and then spreads down the body). Less frequently, retro-orbital pain and gastro-intestinal symptoms, such as abdominal pain, are reported.
Fetal abnormalities
There is strong scientific consensus that pregnant women who become infected with ZIKV can transmit the infection to their unborn babies, with potentially serious consequences.26, 27, 50, 51 Reports from several countries, most notably Brazil, where ZIKV outbreaks have occurred, indicate a coincident increase in cases of congenital abnormalities, some of which are severe, and include microcephaly.24, 25, 52, 53 Based on current evidence, the risk of congenital abnormalities appears to relate to all trimesters of pregnancy.24, 54-57 Additional ongoing research is necessary in order to determine the likelihood and spectrum of adverse fetal outcomes associated with ZIKV infection.
ZIKV infection is not believed to pose a risk of birth defects for future pregnancies.
Guillain-Barré Syndrome (GBS)
GBS is recognised to be a complication of infection with a number of organisms, including Campylobacter spp., influenza virus, Epstein-Barr virus, HIV and Mycoplasma pneumoniae, as well as some non-infectious conditions.
In French Polynesia, after a local ZIKV outbreak starting in 2013, an increase in autoimmune and neurological diseases (including GBS) was observed.8, 58 The incidence of GBS cases during this outbreak was estimated to be 0.24 per 1,000 ZIKV infections.58
There is a strong scientific consensus that ZIKV infection can cause GBS
58-62 and other neurological conditions.45, 63
Persons at increased risk of disease
Susceptibility to primary ZIKV infection is assumed to be universal. It is likely that those who have previously had ZIKV infection have immunity to re-infection.
Disease occurrence and public health significance
Knowledge of the geographical distribution of ZIKV is based on: results of serosurveys; viral isolation studies in mosquitoes and humans; reports of travel-associated cases, and the limited number of published outbreaks. As noted above (under Infectious agent), before 2007, the areas with reported ZIKV circulation included tropical Africa and Southeast Asia.
An outbreak was reported on Yap Island, Federated States of Micronesia (FSM) from April to July 2007.5 Between 2013 and 2015, several significant outbreaks occurred in the Pacific region including French Polynesia.6
In 2015, ZIKV emerged in South America with widespread outbreaks reported initially in Brazil and Columbia,9, 10 with spread to many countries in South and Central America and the Caribbean.
Public Health Emergency of International Concern
On 1 February 2016, the WHO declared a Public Health Emergency of International Concern (PHEIC) to facilitate a globally coordinated response to investigate the suspected association between ZIKV outbreaks and a concurrent increase in congenital abnormalities (including microcephaly) and neurological conditions (including GBS).
64
On 18 November 2016, the WHO declared the end of the PHEIC, with the Emergency Committee on Zika and microcephaly recommending that the alert phase be scaled down to one of long term surveillance and research.65
3. Routine prevention activities
Prevention activities for ZIKV depend on reducing human exposure to vector bites and vector control measures in ZIKV-receptive areas, along with prevention of non-mosquito (e.g. sexual or transfusion-associated) transmission of ZIKV. There is no vaccine.
In north Queensland, and parts of central and southwest Queensland, where the
Ae. aegypti are present, and in the Torres Strait where both
Ae. aegypti and
Ae. albopictus are known to be present, public health vector control teams may respond to reduce the risk of local transmission through insecticide spraying and other vector reduction strategies. Outside these areas, notification and case interviews are the minimum required public health actions.
Travel advice
All travellers are advised to take the following mosquito bite prevention measures when travelling to areas currently affected by ZIKV or wherever mosquito borne diseases are present. These precautions are necessary in the daytime as well as night time.
- Cover as much exposed skin as possible, including wearing light coloured long-sleeved shirts and long pants.
- Use insect repellents, as per manufacturer’s instructions.
- The most effective mosquito repellents contain Diethyl Toluamide (DEET) or Picaridin. Repellents containing oil of lemon eucalyptus (OLE) (also known as Extract of Lemon Eucalyptus) or para menthane diol (PMD) also provide adequate protection.
- Note that insect repellents containing DEET or picaridin, are safe for pregnant and breastfeeding women and children older than 2 months when used according to the product label.
- If both sunscreen and insect repellent are used, sunscreen should be applied before the repellent.
- Use insecticide-treated (such as permethrin) clothing and gear (such as boots, pants, socks, and tents).
- Use bed nets as necessary.
- Stay and sleep in screened-in or air-conditioned rooms.
- Seek medical advice, as soon as practicable, if unwell with a high fever and/or other relevant symptoms during or soon after travel.
People infected with ZIKV should be advised against travelling to the ZIKV-receptive area of Australia until at least a week after illness onset or laboratory confirmation of infection.
Information on ZIKV affected countries can be found at the Australian Department of Health
Zika virus website. Given the difficulties in determining specific locations within countries where there is local ZIKV transmission, this list is likely to change over time.
NSW specific advice
The
World Health Organization (WHO) provides updates on the global epidemiology of Zika virus transmission, including list of affected countries.
Mosquito surveillance and control measures in Australia
ZIKV is not endemic in Australia, but persons infected in Latin America, the Pacific and South-east Asia are known to have travelled to Australia.
For mosquito-borne transmission to occur in Australia, the conditions required include:
- imported cases, viraemic in Australia
- sufficient numbers of efficient local vectors (e.g. Ae. aegypti)
- vectors that live long enough (beyond one extrinsic incubation period) to transmit the virus (dependent on temperature and humidity)
- access to enough people for ongoing transmission cycles with a significant proportion of exposed individuals developing sustained viraemia. It is likely that even asymptomatic cases have some capability to infect mosquitoes, as with dengue,66 though this is uncertain.
Currently the only known vectors for ZIKV in Australia are Ae. aegypti and Ae. albopictus, but Ae. aegypti is currently limited to towns in north, and some parts of central and southwest Queensland. Larvae develop in artificial water-holding containers close to or inside people’s homes (such as buckets, tyres, pot-plant bases, roof gutters and rainwater tanks). Ae. aegypti is a day-biting species, with increased biting activity around sunrise and sunset, with humans being its preferred source of blood meals.
Ae. albopictus, which is not established in mainland Australia (though is being actively confined to the Torres Strait islands), breeds in artificial containers and some naturally occurring sites such as tree holes and coconut shells. Its adults prefer to rest in heavily-shaded outdoor sites and the female takes blood from a range of mammals.
Because cases of ZIKV infection can go undiagnosed, a local outbreak of ZIKV in a receptive area may spread unnoticed before being detected. As local-area vector control around known cases may not be effective, a range of preventive activities are necessary to reduce vector breeding, survival, and biting of humans, with a particular focus on higher-risk premises to prevent ZIKV outbreaks in receptive areas. This may also include vector- control activities and education at sites where pregnant women are likely to be present, such as ante-natal services, child-care facilities and schools.
Water filled containers should be treated with insect growth regulators (e.g. F/methoprene), Bti* or pesticide sprays. Emptying or removing containers (source reduction) is effective but laborious, but not efficient during large outbreaks. The response when an outbreak occurs is outlined in section Special situations (below).
For ZIKV infection cases outside the ZIKV-receptive area in Australia and which are not related to exposures overseas, the vector or other source of infection must be identified promptly.
* Bti - Bacillus Thuringiensis Israelensis
ZIKV and pregnancy
Women who are pregnant (in any trimester) or those who plan to become pregnant are advised to defer travel to high risk countries and consider deferral to moderate risk countries (refer to the Commonwealth Department of Health
Zika virus website).
Women who have been pregnant during travel to ZIKV-affected countries are advised to seek advice from their health care provider. Testing for ZIKV is recommended in these women. Follow up with an obstetric specialist is recommended if ZIKV infection is confirmed. It is not yet possible to quantify the risk of harm that maternal ZIKV infection may pose to the fetus.
Women who are planning or at risk of pregnancy, should be advised to avoid pregnancy during travel to a high or moderate-risk ZIKV country, and to avoid unprotected sex and pregnancy, for at least eight weeks after their arrival home. Similar advice applies to a partner who has also travelled.
For men with a partner who is planning pregnancy or at risk of pregnancy, and who have travelled to a high or moderate-risk ZIKV country, or has a confirmed ZIKV infection, their partner’s pregnancy should be deferred until at least six months after return, or, until six months after the date that ZIKV infection was diagnosed.
In men and women actively planning pregnancy, testing in asymptomatic individuals more than four weeks after returning from a high or moderate-risk ZIKV country may be considered in consultation with their doctor.
Sexual transmission advice
Given the potentially serious implications of sexual transmission of the virus to a pregnant woman and the risk of local transmission in north Queensland, recommendations to reduce this risk of transmission have been developed and can be found in Appendix 1 (below).
Pregnant women should avoid unprotected sex with a male partner who has been to a high or moderate-risk ZIKV-affected country for the duration of the pregnancy, or for six months – whichever is longer.
Pregnant women should avoid unprotected sex with a female partner who has been to a high or moderate-risk ZIKV affected country for the duration of the pregnancy, or at least 8 weeks – whichever is longer.
If a female partner has travelled or been potentially exposed, she should avoid unprotected sex for at least eight weeks after the last day in a high or moderate-risk ZIKV-affected country, or for eight weeks after diagnosis.
If a male partner has travelled or been potentially exposed, he should avoid unprotected sex for at least six months after the last day in a high or moderate-risk ZIKV-affected country if no symptoms appear, or for at least six months from time of diagnosis. Men should not donate sperm for at least six months from the time of last exposure or time of diagnosis.
Further information on risk categories from sexual transmission and travel is available at the Centers of Disease Control and Prevention
Countries and Territories at risk for Zika, and
Recommendations for Travellers and People living abroad). Further information on reducing the risk of sexual transmission refer to Appendix 1: Table of recommendations regarding ZIKV virus prevention.
Blood donation
Deferral
People who have been to a ZIKV-affected country should defer donation of blood for four weeks after they have returned.
A person diagnosed with ZIKV infection should be advised that they cannot donate blood for a minimum of 4 weeks after recovery from all symptoms.
A sexual contact of a person diagnosed with ZIKV infection should be advised that they cannot donate blood for a minimum of 4 weeks after sexual contact with someone who:
- has current ZIKV infection or
- has recovered from ZIKV infection in the preceding three months.
For the latest information, refer to the
Australian Red Cross Blood Service.
Blood Service notification
The PHU should notify the Blood Service when an outbreak of ZIKV has been identified in their area. .
4. Surveillance objectives
- To detect and enable prompt response to:
- imported cases of Zika into receptive areas of Australia (to prevent local transmission)
- locally acquired cases of Zika in Australia
- imported cases of Zika anywhere in Australia (to provide advice on issues such as pregnancy, preventing sexual transmission, travel, etc.).
- To monitor the epidemiology of Zika to inform development of prevention and control strategies.
5. Data management
NSW specific advice
Both confirmed and probable cases should be entered into NCIMS within one working day of notification. Nationally, cases are currently classified under the designation “flavivirus infection (unspecified)". In NSW, cases are entered under 'Zika' and the Place of Acquisition is completed in the NCIMS Clinical module.
6. Communications
NSW specific advice
For confirmed and probable cases believed to have been either acquired in, or who have travelled to Australia's ZIKV-receptive area during their exposure and/or infectious period, the PHU should notify NSW One Health Branch as soon as possible which will facilitate notification to Queensland Health Communicable Disease Branch.
For confirmed or probable cases imported from overseas and diagnosed in non-receptive areas, routine data entry is sufficient. Case reporting to NSW One Health Branch should be completed in the NCIMS Zika event questions and include case details, onset date, place of acquisition, pregnancy status and laboratory findings as described below.
For confirmed or probable cases in pregnant women, the NSW One Health Branch should be notified as soon as possible.
7. Case definition
NSW specific advice
Zika virus case definition
Confirmed and probable cases are nationally notifiable under the disease
Flavivirus infection
(unspecified). In NSW, Zika virus infection and congenital Zika virus infection are separate conditions in NCIMS.
Reporting
Both confirmed and probable cases are nationally notifiable. Both confirmed and probable cases should be further sub-classified into clinical and non-clinical cases.
Confirmed case
A confirmed case requires laboratory definitive evidence only. Clinical evidence should be used to sub-classify cases as clinical or non-clinical.
Laboratory definitive evidence
- Detection of ZIKV by nucleic acid testing or virus isolation
or
- IgG seroconversion or a significant increase in antibody level or a fourfold or greater rise in titre of ZIKV-specific IgG, and recent infection by dengue or other epidemiologically possible flaviviruses has been excluded
or
- detection of ZIKV-specific IgM in cerebrospinal fluid, in the absence of IgM to other possible infecting flaviviruses.
Probable case
A probable case requires laboratory suggestive evidence
and epidemiological evidence. Clinical evidence should be used to sub-classify cases as clinical or non-clinical.
Laboratory suggestive evidence
Detection of ZIKV-specific IgM in the absence of IgM to other epidemiologically possible flaviruses or flavivirus vaccination in the 3 weeks prior to testing.
Notes:
- If the date of most recent exposure was greater than 4 weeks before the specimen date, then ZIKV-specific IgG must also be positive.
- If ZIKV-specific IgG was initially negative and subsequent testing greater than 4 weeks after exposure fails to demonstrate seroconversion the case should be rejected.
Epidemiological evidence
Clinical case
- Travel to or residence in a ZIKV receptive country* or area in Australia within two weeks prior to symptom onset
or
- sexual exposure to a confirmed or probable case of ZIKV infection within two weeks prior to symptom onset.
Non-clinical case
- Travel to or residence in a ZIKV receptive country* or area in Australia within two months prior to specimen date
or
- sexual exposure to a confirmed or probable case of ZIKV infection within two months prior to specimen date.
Clinical case
Both confirmed and probable cases should be further sub-classified into clinical or non-clinical cases.
Clinical evidence
An acute illness within 2 weeks of exposure with 2 or more of the following symptoms:
- fever
- headache
- myalgia
- arthralgia
- rash
- non-purulent conjunctivitis.
In the absence of clinical evidence, the case will be classified as non-clinical.
*ZIKV receptive countries and areas are outlined on the
Global Consensus Map. Areas are considered receptive to ZIKV where the likelihood of local acquisition is placed on the map as ‘uncertain’ or more.
Congenital Zika virus infection case definition
Confirmed and probable cases are nationally notifiable under the disease Flavivirus infection (unspecified) using the Organism Name field to specify congenital ZIKV infection.
Reporting
Both confirmed and probable cases are nationally notifiable.
Confirmed case
A confirmed case requires laboratory definitive evidence only.
Laboratory definitive evidence
Fetal (at 20 weeks gestation or more)
Isolation or detection of ZIKV from appropriate clinical samples (i.e. fetal blood, amniotic fluid, chorionic villus sample or post-mortem cerebrospinal fluid or tissue) by viral culture or nucleic acid testing.
Infant (within 28 days following birth)
Isolation or detection of ZIKV from appropriate clinical samples by viral culture or nucleic acid testing, with no history of travel since birth to, or residence in, a ZIKV receptive country* or area in Australia.
Probable case
A probable case requires clinical evidence AND epidemiological evidence.
Clinical evidence
Microcephaly
a-e or other CNS abnormalities
f in the infant or fetus (in the absence of any other known cause).
Epidemiological evidence
Confirmed or probable ZIKV infection in the mother during pregnancy.
* ZIKV receptive countries and areas are outlined on the
Global Consensus Map. Areas are considered receptive to ZIKV where the likelihood of local acquisition is placed on the map as ‘uncertain’ or more.
aHead circumference <-2SD below mean for gestation.
bWHO Assessment of infants with microcephaly in the context of ZIKV. Interim guidance. 4 March 2016, WHO/ZIKV/MOC/16.3 Rev.1.
c WHO Growth standards for term neonates
dWHO Pregnancy management in the context of ZIKV. Interim guidance. 2 March 2016. WHO/ZIKV/MOC/16.2
eIntergrowth standards for preterm neonates (Villar, José et al. (2014). International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet; (384). 9946: 857–868).
fThese include: ventriculomegaly, calcifications, abnormal sulcation and gyration, brain atrophy, callosal dysgenesis, microophthalmia, eye calcifications.
8. Laboratory testing
Testing guidelines
Testing for ZIKV is recommended in persons who have a clinically compatible illness and have travelled to an area with known ZIKV activity during the exposure period.
ZIKV testing is performed at state public health laboratories in Australia. If ZIKV infection is suspected, clinicians are advised to discuss testing with their local pathology provider. Testing for ZIKV infection may include IgM, IgG serology and PCR performed on blood, urine, amniotic fluid, cerebrospinal fluid or fetal tissues as appropriate.
Acute serum (taken soon after exposure or symptom appearance) and convalescent serum (two weeks later), should be taken wherever possible. The paired samples are important for parallel testing to:
- confirm recent ZIKV infection (especially in ZIKV PCR-negative cases);
- confirm or rule out past flavivirus infections; and
- help exclude false positive IgM and IgG tests due to cross reactivity with similar viruses such as dengue.
Pathology requesters should provide on the request form, details of overseas travel and clinical history (including symptom onset date), to help direct appropriate laboratory testing. Symptom onset date is extremely important to ensure that the most appropriate test is performed. Details of any previous flavivirus vaccination (e.g. Japanese encephalitis, yellow fever) or previous flavivirus illness (e.g. West Nile virus, dengue), can be useful for the pathologist in test interpretation.
Because they have similar geographic distribution(s) and symptoms, dengue and chikungunya virus infection should be considered for patients with suspected ZIKV infections. They should also be evaluated for other common causes of fever and rash in a traveller who has returned from overseas or a ZIKV-receptive area in Australia.
For further information, please refer to the Commonwealth Department of Health
Information about Zika virus testing.
9. Case management
A public health unit checklist can be found at Appendix 3.
Response times
Investigation should begin on the day of notification of a confirmed or probable case, in order to determine whether a response is required from the local PHU to help prevent local transmission. If any case is suspected to have been acquired in an Australian jurisdiction but diagnosed in a different jurisdiction, the communicable diseases branch in the jurisdiction of presumed acquisition should be notified on the same day to ensure that case/contact management and mosquito-control measures can begin promptly.
NSW specific advice
For confirmed and probable cases believed to have been acquired in, or who have travelled to, the ZIKV-receptive area in Australia during their exposure and/or infectious period, the PHU should immediately notify the NSW One Health Branch which will facilitate information to Queensland Health.
Response procedure
Case investigation
Investigate all confirmed and probable cases using the Example Zika virus infection case investigation form (Appendix 4) or similar jurisdictional-specific form.
PHU staff should ensure that action has been taken to:
- confirm the onset date, travel history and symptoms of ZIKV infection (if any)
- confirm results of laboratory tests
- where possible seek the doctor’s consent to contact the case or relevant care-giver
- interview the case or carer and determine the country of acquisition and likely route of transmission and
- provide advice about risks of transmission ( e.g. sexual, blood donation, mosquito-borne, pregnancy implications), as applicable to the circumstance of the case.
For probable cases, advise the treating doctor of the need to obtain a convalescent serum sample 2-3 weeks after onset, or if asymptomatic 2-3 weeks after initial testing, to be sent to the same public health laboratory for testing in parallel with the acute sample.
Consider potential differential diagnoses of fever and rash in a returned traveller, see Testing guidelines under section 8. Laboratory testing.
Exposure Investigation
Determine whether the case had travelled to known or possible ZIKV affected areas during the exposure period (3-12 days prior to onset of symptoms or up to 2 weeks prior to laboratory confirmation by PCR of ZIKV infection).
Where a case has been diagnosed based on serology, it may not be possible to determine the viraemic period.
If the case has not travelled to a known ZIKV affected area during the exposure period consider local transmission of ZIKV. Determine if the case has recently received any blood products or if any of their sexual contacts have a recent history of travel to a ZIKV-affected country or the ZIKV-receptive area of Australia, and therefore might be an unidentified source case.
If local transmission is suspected, immediately notify the communicable diseases branch in the state or territory of acquisition to arrange for expert investigation and control advice.
If a case who is considered likely to be viraemic stayed in, or travelled to, the ZIKV-receptive area of Australia:
- identify where the case lived/worked/visited while viraemic and
- notify the communicable diseases branch in the state or territory of diagnosis as soon as possible so that case information can be shared with health officials responsible for communicable disease control in the ZIKV-receptive jurisdiction (currently only Queensland).
Case treatment
No specific treatment is available for ZIKV infection. Treatment is generally supportive and may include rest, fluids, and use of analgesics and antipyretics. Aspirin and other NSAIDs should be avoided until dengue can be excluded to reduce the risk of haemorrhage.
Education
Cases should be provided with the Zika virus fact sheet (Appendix 2) and advised to seek medical attention if symptoms worsen.
Isolation and restriction
Infected people (confirmed and probable cases) should be advised against travelling to the ZIKV-receptive area of Australia until at least a week after the onset of their illness or laboratory confirmation of ZIKV infection.
People with ZIKV infection should be advised to take particular precautions against being bitten by mosquitoes (where relevant) for up to a week from onset of illness or laboratory confirmation of ZIKV infection to reduce the risk of local vector-borne transmission. In Australia, this is particularly relevant to cases in Queensland.
Additional information specifically relating to reducing sexual transmission and blood donation transmission can be found under section 3. Routine prevention activities.
Pregnancy management
Pregnant women with confirmed or probable ZIKV infection should be referred to a suitably qualified expert in diagnosis and management of the consequences of perinatal infections (e.g. obstetrician or maternal fetal medicine specialist).
Active case finding in the ZIKV-receptive area of Australia
Active case finding should occur following identification of a case acquired or present while viraemic in the ZIKV-receptive area of Australia. The purpose is to quickly establish whether an outbreak is occurring, or could occur, and direct control efforts to areas where vectors may be or become infective. The methods used to actively find more cases include:
- active case finding among the household contacts and residents in the exposure area, and advising those with symptoms consistent with ZIKV to see their doctor for appropriate ZIKV testing or arranging testing directly
- considering testing of asymptomatic co-travellers, household contacts, and pregnant women in exposure area
- alerting general practitioners, laboratories and hospital emergency departments to the occurrence of a local case, and requesting them to consider and test for ZIKV infection in recent and future cases with compatible symptoms and ensuring appropriate ZIKV tests are ordered and
- providing information to institutional settings such as schools, aged care facilities, prisons and workplaces in the exposure area.
10. Environmental evaluation and response in the ZIKV-receptive area
Evaluating the risk for further ZIKV transmission should be coordinated by the PHU in consultation with the medical entomologist and/or local environmental health authority to:
- identify areas where transmission is possible
- actively search for any further cases and
- direct mosquito elimination activities.
Vector surveillance and control activities should be undertaken according to local procedures and guidelines.
A departure from a dengue response would take into consideration the possible wider spread by asymptomatic cases, the potential for sexual transmission, and specific measures required to protect pregnant women from vector mosquitoes.
11. Contact management
Pregnant women
Routine contact management seeks to identify pregnant women at increased risk of ZIKV infection, either from:
- travel to a high or moderate risk ZIKV country
- co-travel with a confirmed or probable case (so potentially exposed to infective mosquitoes)
- as a sexual partner of a confirmed or probable case while the case is potentially infectious and
- in the ZIKV-receptive area of Australia, possible local vector-borne transmission.
Pregnant women who are sexual partners of a confirmed or probable case should be referred for specialist obstetric assessment and ZIKV testing. If found to fit the definition of a confirmed or probable case, their infection should be notified and investigated as per section 9. Case management.
Infants born to mothers infected with ZIKV
Infants born to mothers who were infected with ZIKV during their pregnancy should be tested for ZIKV infection and be referred for specialist paediatric assessment.
Australian ZIKV-receptive area
Additional contact management is required when the disease is acquired or thought to have been acquired in the Australian ZIKV-receptive area OR when the disease is acquired overseas and the case was either a resident or visitor in the Australian ZIKV-receptive area during the period of possible viraemia.
This includes all people who are epidemiologically linked by having the same potential exposure as the case, including household members and persons who work or travelled with the case. Follow-up of these contacts is also part of active case finding (see section 9. Case management) and might lead to the identification of other imported or locally-acquired cases.
Other contacts
If other co-travellers are identified with symptoms consistent with a ZIKV infection they should be referred for ZIKV testing. There may be circumstances where asymptomatic sexual contacts and co-travellers are referred for testing.
Potential exposure to ZIKV infection through receipt of blood products or from sharing of non-genital body fluids is likely to be a rare indication for contact tracing in Australia. Additional information specifically relating to reducing sexual transmission and blood donation deferral can be found under section 3. Routine prevention activities.
Prophylaxis
Chemo-prophylaxis not applicable.
Education
As with case management, contacts should be provided with the Zika virus fact sheet (Appendix 2) and advised to seek medical attention if symptoms worsen.
Isolation and restriction
Not applicable unless symptomatic, then the same precautions apply as found in section 9. Case management.
12. Special situations
Outbreaks
ZIKV is not endemic to Australia but there is a risk, as with dengue, of local outbreaks in areas with competent mosquito vectors (i.e. the ZIKV-receptive area) beginning with a single imported case.
A confirmed case of locally acquired vector-borne ZIKV infection anywhere in Australia requires an outbreak response. An outbreak of ZIKV infection should be managed as per local procedures and guidelines.
In an outbreak, an incident management team including a medical entomologist and local government representatives should be formed urgently and the outbreak managed according to local outbreak management plans.
Some additional issues the PHU should address during an outbreak are:
- informing the wider public health network, local government and relevant Australian Government bodies (e.g. National Incident Room, Department of Agriculture) of the outbreak to increase surveillance and prepare for outbreak response;
- communicating regularly with general practitioners, hospital emergency departments and local pathology laboratories to ask them to be alert for ZIKV cases;
- collecting and maintaining epidemiological information on cases e.g. timeline and mapping of cases;
- regularly communicating with medical entomologists and agencies charged with vector control responsibilities so that vector control measures can be appropriately directed;
- case finding in areas of ZIKV transmission may become the priority as the outbreak develops;
- using media alerts and updates to communicate key messages during the outbreak; and
- liaising with the Australian Red Cross Blood Service as blood donation services in outbreak locations may be restricted.
13. References
- State of Queensland (Queensland Health). Queensland dengue management plan 2015–2020, 2015.
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952;46(5):509-520.
- Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg 1954;48(2):139-145.
- Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, et al. Genetic characterization of Zika virus strains: geographic expansion of the Asian lineage. PLoS Neglected Tropical Disseases 2012;6(2):e1477.
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. New England Journal of Medicine 2009;360(24):2536-2543.
- Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, et al. Zika virus, French polynesia, South pacific, 2013. Emerging Infectious Diseases 2014;20(6):1085-1086.
- European Centre for Disease Prevention and Control. Rapid risk assessment: Zika virus infection outbreak, French Polynesia. Stockholm: ECDC; 2014.
- Oehler E, Watrin L, Larre P, Leparc-Goffart I, Lastere S, Valour F, et al. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill 2014;19(9).
- Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerging Infectious Diseases 2015;21(10):1885-1886.
- World Health Organization (WHO). Zika virus outbreaks in the Americas. Wkly Epidemiol Rec 2015;90(45):609-610.
- Boorman JP, Porterfield JS. A simple technique for infection of mosquitoes with viruses; transmission of Zika virus. Trans R Soc Trop Med Hyg 1956;50(3):238-242.
- Haddow AJ, Williams MC, Woodall JP, Simpson DI, Goma LK. Twelve Isolations of Zika Virus from Aedes (Stegomyia) Africanus (Theobald) Taken in and above a Uganda Forest. Bull World Health Organ 1964;31:57-69.
- Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti mosquitoes in Malaysia. The American Journal of Tropical Medicine and Hygiene, 1969;18(3):411-415.
- Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Neglected Tropical Disseases 2013;7(8):e2348.
- Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Neglected Tropical Diseases 2014;8(2):e2681.
- Fagbami AH. Zika virus infections in Nigeria: virological and seroepidemiological investigations in Oyo State. Journal of Hygiene 1979;83(2):213-219.
- Savage HM, Ledermann JP, Yug L, Burkhalter KL, Marfel M, Hancock WT. Incrimination of Aedes (Stegomyia) hensilli Farner as an epidemic vector of Chikungunya virus on Yap Island, Federated States of Micronesia, 2013. American Journal of Tropical Medicine and Hygiene 2015;92(2):429-436.
- Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, et al. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Neglected Tropical Disseases 2014(1935-2735 (Electronic)).
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 2014;20(10):O595-596.
- Sarno M, Sacramento GA, Khouri R, do Rosario MS, Costa F, Archanjo G, et al. Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Neglected Tropical Disseases 2016;10(2):e0004517.
- Jouannic JM, Friszer S, Leparc-Goffart I, Garel C, Eyrolle-Guignot D. Zika virus infection in French Polynesia. Lancet 2016.
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, et al. Zika Virus Associated with Microcephaly. New England Journal of Medicine 2016;374(10):951-958.
- Driggers RW, Ho CY, Korhonen EM, Kuivanen S, Jaaskelainen AJ, Smura T, et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. New England Journal of Medicine 2016.
- Brasil P, Pereira JP, Jr., Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med 2016.
- Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, do Carmo GM, Henriques CM, Coelho GE, et al. Increase in Reported Prevalence of Microcephaly in Infants Born to Women Living in Areas with Confirmed Zika Virus Transmission During the First Trimester of Pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep 2016;65(9):242-247.
- de Oliveira CS, da Costa Vasconcelos PF. Microcephaly and Zika virus. J Pediatr (Rio J) 2016;92(2):103-105.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects - Reviewing the Evidence for Causality. N Engl J Med 2016.
- Dupont-Rouzeyrol M, Biron A, O'Connor O, Huguon E, Descloux E. Infectious Zika viral particles in breastmilk. Lancet 2016.
- World Health Organization (WHO).
Breastfeeding in the context of Zika virus - interim guidance. 2016. Accessed on 22/04/2016.
- McCarthy M. Zika virus was transmitted by sexual contact in Texas, health officials report. BMJ 2016;352.
- Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerging Infectious Diseases 2011;17(5):880-882.
- Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerging Infectious Diseases 2015;21(2):359-361.
- Atkinson B, Hearn P, Afrough B, Lumley S, Carter D, Aarons EJ, et al. Detection of Zika virus in semen [letter]. Emerging Infectious Diseases 2016;May.
- Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, et al. Male-to-Male Sexual Transmission of Zika Virus — Texas, January 2016. MMWR Morb Mortal Wkly Rep 2016;65:372-374.
- D'Ortenzio E, Matheron S, de Lamballerie X, Hubert B, Piorkowski G, Maquart M, et al. Evidence of Sexual Transmission of Zika Virus. New England Journal of Medicine 2016.
- Freour T, Mirallie S, Hubert B, Splingart C, Barriere P, Maquart M, et al. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill 2016;21(23).
- Brooks RB, Carlos MP, Myers RA, White MG, Bobo-Lenoci T, Aplan D, et al. Likely Sexual Transmission of Zika Virus from a Man with No Symptoms of Infection - Maryland, 2016. MMWR Morb Mortal Wkly Rep 2016;65(34):915-916.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected Female-to-Male Sexual Transmission of Zika Virus - New York City, 2016. MMWR Morb Mortal Wkly Rep 2016;65(28):716-717.
- Turmel JM, Abgueguen P, Hubert B, Vandamme YM, Maquart M, Le Guillou-Guillemette H, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet 2016;387(10037):2501.
- Mansuy JM, Pasquier C, Daudin M, Chapuy-Regaud S, Moinard N, Chevreau C, et al. Zika virus in semen of a patient returning from a non-epidemic area. Lancet Infectious diseases 2016;16(8):894-895.
- Mansuy JM, Suberbielle E, Chapuy-Regaud S, Mengelle C, Bujan L, Marchou B, et al. Zika virus in semen and spermatozoa. Lancet Infect Dis 2016;16(10):1106-1107.
- Barzon L, Pacenti M, Franchin E, Lavezzo E, Trevisan M, Sgarabotto D, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill 2016;21(32).
- Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill 2016;21(32).
- Visseaux B, Mortier E, Houhou-Fidouh N, Brichler S, Collin G, Larrouy L, et al. Zika virus in the female genital tract. Lancet Infect Dis 2016;16(11):1220.
- Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika Virus Infection in the Central Nervous System and Female Genital Tract. Emerg Infect Dis 2016;22(12):2228-2230.
- Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, Shan Yan A, Cao-Lormeau VM, Broult J. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014 . Euro Surveill. 2014;19(14):pii=20761.
- Motta IJ, Spencer BR, Cordeiro da Silva SG, Arruda MB, Dobbin JA, Gonzaga YB, et al. Evidence for Transmission of Zika Virus by Platelet Transfusion. N Engl J Med 2016;375(11):1101-1103.
- Barjas-Castro ML, Angerami RN, Cunha MS, Suzuki A, Nogueira JS, Rocco IM, et al. Probable transfusion-transmitted Zika virus in Brazil. (1537-2995 (Electronic)).
- Lessler JT, Ott CT, Carcelen AC, Konikoff JM, Williamson J, Bi Q, et al. Times to key events in the course of Zika infection and their implications: a systematic review and pooled analysis. Bulletin of the World Health Organization 2016;94(4).
- Cuevas EL TV, Rozo N, et al. Preliminary Report of Microcephaly Potentially Associated with Zika Virus Infection During Pregnancy — Colombia, January–November 2016. MMWR Morb Mortal Wkly Rep 2016.
- Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, Amorim MM, Batista AG, Chimelli L, et al. Congenital Brain Abnormalities and Zika Virus: What the Radiologist Can Expect to See Prenatally and Postnatally. Radiology 2016;281(1):203-218.
- van der Linden V PA, Dobyns W, et al. Description of 13 Infants Born During October 2015–January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth — Brazil. MMWR Morb Mortal Wkly Rep 2016;65:1343-1348.
- Barcellos C XD, Pavão A, Boccolini C, Pina M, Pedroso M, et al. . Increased Hospitalizations for Neuropathies as Indicators of Zika Virus Infection, according to Health Information System Data, Brazil. Emerg Infect Dis 2016;22(11):1894-1899.
- Pacheco O, Beltran M, Nelson CA, Valencia D, Tolosa N, Farr SL, et al. Zika Virus Disease in Colombia - Preliminary Report. N Engl J Med 2016.
- Johansson MA, Mier-y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the Risk of Microcephaly. N Engl J Med 2016;375(1):1-4.
- Jaenisch T, Rosenberger KD, Brito C, Brady O, Brasil P, Marques ETA.
Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull World Health Organ 2017;95:191–198.
- Franca GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 2016;388(10047):891-897.
- Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet (1474-547X (Electronic)).
- Rozé B, Najioullah F, Fergé J, Apetse K, Brouste Y, Cesaire R, Fagour C, Fagour L, Hochedez P, Jeannin S, Joux J, Mehdaoui H, Valentino R, Signate A, Cabié A, on behalf of the GBS Zika Working Group. Zika virus detection in urine from patients with Guillain-Barré syndrome on Martinique, January 2016. Euro Surveill. 2016;21(9):pii=30154.
- World Health Organization (WHO). Zika virus, Microcephaly and Guillain-Barré syndrome - situation report 14 April 2016; 2016.
- World Health Organization (WHO). Zika virus, Microcephaly and Guillain-Barré syndrome - situation report 10 March 2016; 2016.
- Dirlikov E, Major CG, Mayshack M, Medina N, Matos D, Ryff KR, et al. Guillain-Barre Syndrome During Ongoing Zika Virus Transmission - Puerto Rico, January 1-July 31, 2016. MMWR Morb Mortal Wkly Rep 2016;65(34):910-914.
- Medina MT, England JD, Lorenzana I, Medina-Montoya M, Alvarado D, De Bastos M, et al. Zika virus associated with sensory polyneuropathy. J Neurol Sci 2016;369:271-272.
- World Health Organization (WHO).
WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. 1 February 2016. Accessed on 17 March 2016.
- World Health Organization (WHO). Fifth meeting of the Emergency Committee under the International Health Regulations (2005) regarding microcephaly, other neurological disorders and Zika virus. 2016. Accessed on 20 February 2017. Available from:
www.who.int/mediacentre/news/statements/2016/zika-fifth-ec/en/
- Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, Long KC, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proceedings of the National Academy of Sciences 2015;112(47):14688-14693.
14. Further information and resources
15. Appendices
Appendix 1:
Table of recommendations regarding ZIKV
Appendix 2:
Zika Virus Fact Sheet
Appendix 3:
PHU checklist in CDNA Zika SoNG
Appendix 4: Zika virus infection case investigation forms
16. Jurisdiction specific issues